Unit 6.2: Energy Diagrams
Identify reactants and products as being higher in energy on an energy
diagram.
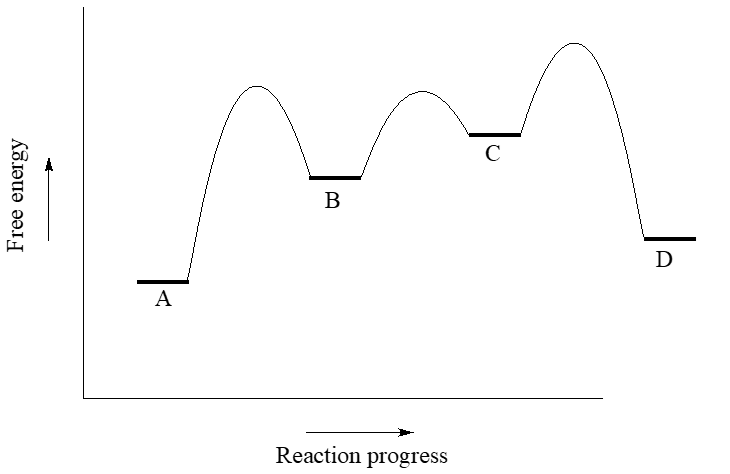
Image credit:
libretexts
-
Does the energy diagram above show a endothermic or exothermic
reaction. Justify your answer.
-
Label the reactants, intermediates, and products on the energy
diagram.
Determine if a process is endothermic or exothermic from an energy
diagram.
-
Given an energy diagram, identify if the reaction absorbs or releases
energy.
-
Explain how the difference in energy between reactants and products
indicates endothermicity or exothermicity.
Calculate ∆H from values on an energy diagram.
-
Using a diagram with energy levels of reactants and products,
calculate ∆H.
-
If reactants are at 150 kJ/mol and products at 80 kJ/mol, what is ∆H?
