Select the correct wavelength for maximum absorbance based on absorbance vs. wavelength graphs.
- Use the absorption spectrum below to determine the wavelength a spectrophotometer should be set to determine the concentration of chlorophyll A.
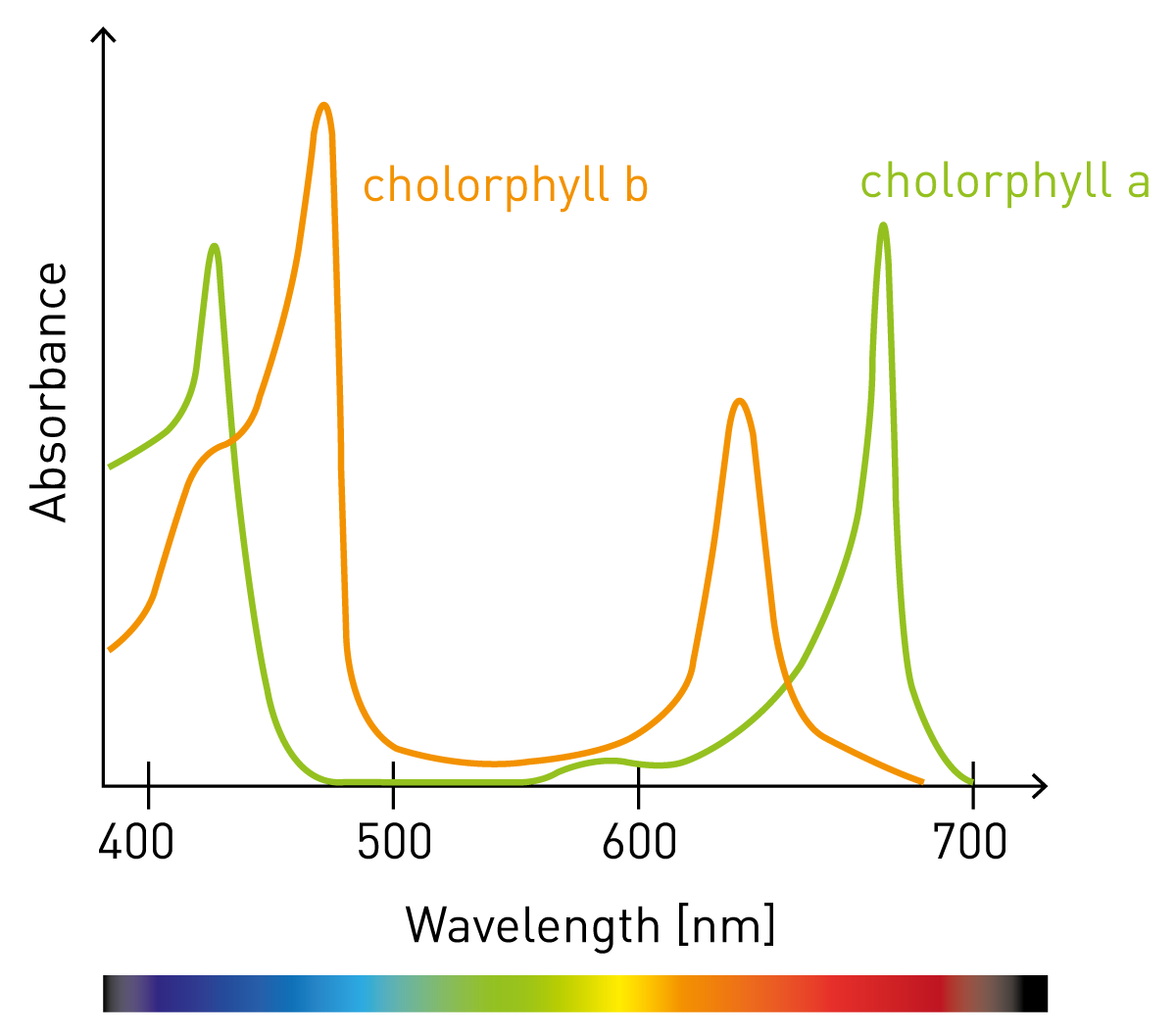
Image credit: BMG LABTECH – Absorbance